Philips HeartStart AED - Onsite Automated External Defibrillator


Philips HeartStart AED - Onsite Automated External Defibrillator
0 item is in your cart. View cart now
All product and company names are trademarks of their respective holders. Use of them does not imply any affiliation with or endorsement or sponsorship by them.
Details
The Philips HeartStart biphasic AED (Automatic External Defibrillator) can be used on patients of any age, with the use of pedatric or adult defibrillation pads. The HeartStart has an optional training pad cartridge so staff may practice their AED skills and become familiar with the unit. This Philips AED automatically tests all parts, including disposable pads, daily. The HeartStart weighs 3.3 lbs with battery and pads / 2.4 lbs without.
HeartStart Benefits
- Lightweight: Fully equipped at just 3.3 pounds.
- Intuitive: Clean design and clear voice instructions instill the confidence that's needed when administering therapy to a person in cardiac arrest.
- Every-Ready: Powered by a long life (4-year) disposable battery. Automated tests help ensure readiness.
- Versatile: Available for use on anyone of any age, including children and infants.
- Effective: Patented SMART Analysis heart rhythm assessment and SMART Biphasic defibrillation therapy, clinically proven in nearly 10 years of use. No other biphasic waveform is as well documented.
Easy to Use
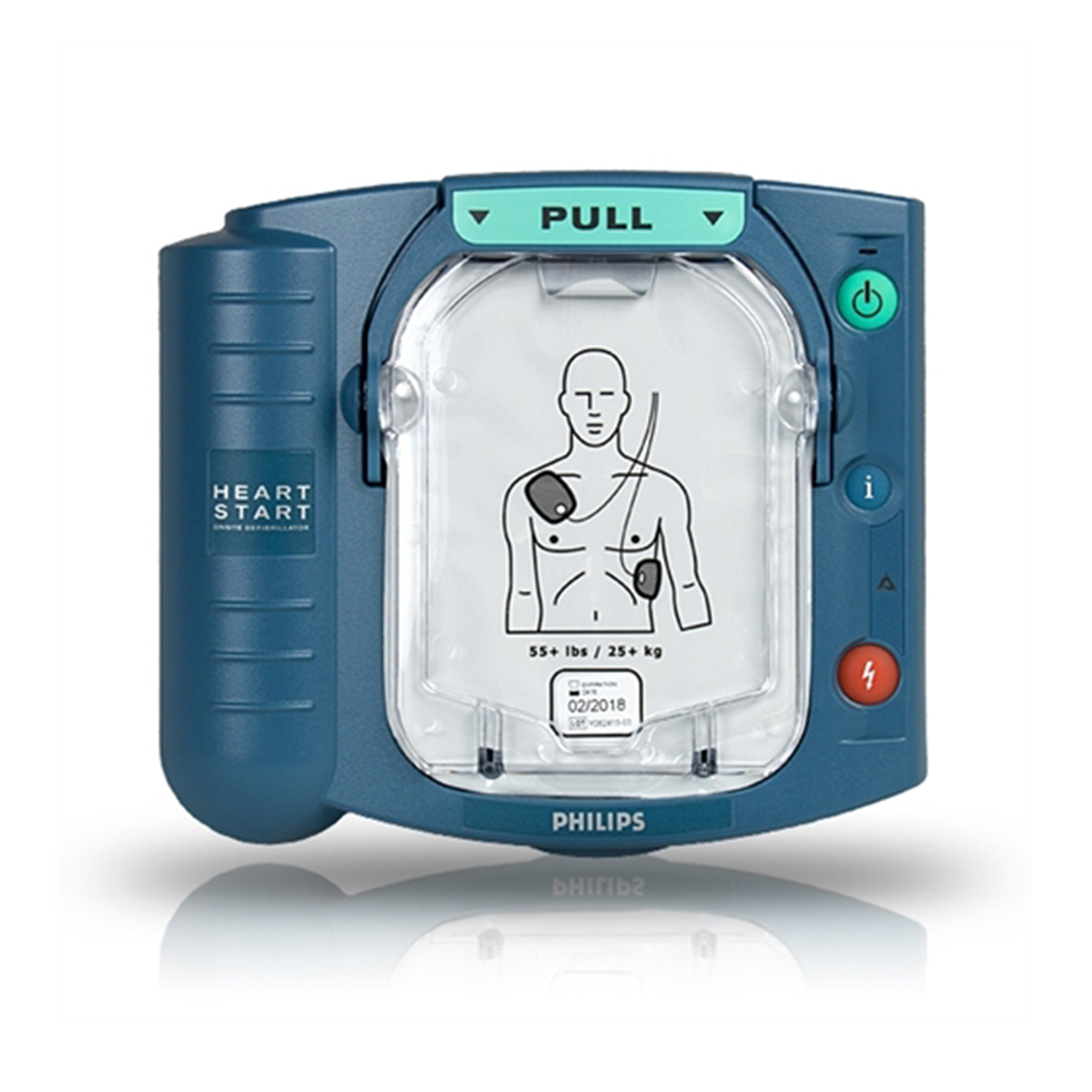
Using the HeartStart OnSite Defibrillator is simple. Pulling the green handle powers-up the defibrillator and activates voice instructions. These instructions are paced to your actions, to help guide you through the entire process, from placing pads on the patient to delivering a defibrillation shock.
HeartStart OnSite determines if a shock is needed, based on its automated assessment of the victim's heart rhythm:
- If a shock is needed, the defibrillator directs you to press the flashing orange "Shock" button. Then, HeartStart OnSite delivers a dose of low-energy biphasic therapy, a highly effective defibrillation waveform that is also gentle to the heart.
- If a shock is not needed, the OnSite Defibrillator instructs you to assess the patient and to perform CPR when necessary. While performing CPR, the defibrillator's voice instructions can be activated to coach you on the frequency and depth of compressions.
HeartStart OnSite also reminds you to call emergency medical services (EMS). And, should EMS need a summary of care, it can be retrieved from the defibrillator's internal memory. An EMS provider simply presses the "i-button" and HeartStart Onsite verbally recounts events from its last clinical use.
Replacable SMART Pads Cartridges
The cartridge contains two adhesive pads that are peeled and placed on the patient's bare skin as indicated by the pictures on the pads. The pads are "smart" because they can sense that they have been removed from the cartridge and applied to the patient.
HeartStart OnSite can be used on patients of any age. Special Infant/Child SMART Pads reduce the defibrillator's shock energy, so that you can treat an infant or child suffering sudden cardiac arrest. The Infant/Child Pads cartridge is marked with an indication of the appropriate weight and with a teddy bear icon for ready identification.
To practice your skills, a special training pads cartridge (adult or infant/child) can be installed in the defibrillator. It suspends the defibrillator's ability to shock, while walking you through patient care scenarios.
Specifications
Defibrillator
- Defibrillator Family: HS1
- How Supplied: Defibrillator, Owner's Manual, battery, 1 adult SMART Pads cartridge, Quick Reference Guide and Quick Start poster
- Waveform: Truncated Exponential Biphasic.Waveform parameters adjusted as a function of each patient's impedance.
- Energy: Single energy output. Adult: nominal 150 Joules into a 50 ohm load. Infant/Child: nominal 50 Joules into a 50 ohm load. Automatically set based on type of SMART Pads cartridge installed.
- Shock-to-Shock Cycle Time: Typically less than 20 seconds between shocks in a series.
- Quick Shock: Able to deliver a shock after the end of a CPR interval, typically in eight seconds.
- Voice Instructions: Detailed voice messages guide responder through use of the defibrillator.
- CPR Coaching: Instructions for adult and infant/child CPR available at user's option.
- Shock Delivery: Via adhesive pads placed on patient’s bare skin as illustrated on pads.
- Controls: Green SMART Pads cartridge handle, green On/Off button, blue i-button, orange Shock button
- Indicators: Ready light; blue i-button; caution light
Physical Weight & Dimensions
- Height: 2.8 inches (7 cm)
- Width: 8.3 inches (21 cm)
- Depth: 7.4 inches (19 cm)
- Weight
- With battery and pads case: 3.3 lbs (1.5 kg)
- Without battery or pads case: 2.4 lbs (1 kg)
Environmental/Physical Requirements
- Sealing: Solid objects per EN60529 class IP2X Drip-proof per EN60529 class IPX1
- Temperature Operating:
- 32° - 122°F (0° - 50°C)
- Standby: 50º - 109°F (10° - 43°C)
- Humidity Operating:
- 0% to 95% relative, non-condensing
- Standby: 0% to 75% relative, non-condensing
- Altitude:
- Operating: 0 to 15,000 feet
- Standby: 0 to 8,500 feet > 48 hours and 8,500 to 15,000 feet < 48 hours
- Shock/Drop Abuse: Withstands 1 meter drop to any edge, corner or surface.
- Vibration: Meets EN1789 random and swept sine, road ambulance specification in operating and standby states.
- EMI (Radiated/Immunity): Meets EN55011 Group 1 Level B Class B and EN61000-4-3.
Patient Analysis System
- Patient Analysis: Evaluates patient ECG to determine if a rhythm is shockable. Rhythms considered shockable are ventricular fibrillation (VF) and certain ventricular tachycardias (VT) associated with lack of circulation. For safety reasons, some VT rhythms associated with circulation will not be interpreted as shockable, and some very low-amplitude or low-frequency rhythms will not be interpreted as shockable VF.
- Sensitivity/Specificity: Meets AAMI DF80 guidelines and AHA recommendations for adult defibrillation (Circulation 1997;95:1677-1682).
- Artifact Detection: The effects of pacemaker artifact and electrical noise are minimized with artifact detection.
Battery (M5070A)
- Type: Volt DC, 4.2 Ah, composed of disposable long-life lithium manganese dioxide primary cells.
- Capacity: Minimum 200 shocks or 4 hours of operating time (EN 60601-2-4:2003)
- Install-by Date: Battery is labeled with an install-by date of at least five years from date of manufacture.
- Standby Life: Four years typical when battery is installed by the install-by date. (Will power the AED in standby state within the specified standby temperature range, assuming one battery insertion test and no defibrillation uses.)
SMART Pads
- Adult SMART Pads Cartridge: M5071A defibrillation pads for patients 8 years of age and older or 55 lbs (25 kg) and over.
- Infant/Child SMART Pads Cartridge: M5072A defibrillation pads for patients under 8 years of age or 55 lbs. (25 kg). Rx only.
- How Supplied: Disposable cartridge, containing adhesive defibrillation pads, clicks into defibrillator for an integrated pads solution.
- Energy Delivered:
- Adult: nominal 150 Joules into a 50 ohm load
- Infant/Child: nominal 50 Joules into a 50 ohm load
Training Pads
- Adult Training Pads Cartridge: M5073A
- Infant/Child Training Pads Cartridge: M5074A
- Function: Special pads put HeartStart OnSite into training mode and disable its energy delivery capability.Training pads feature 8 real-world training scripts. Used with training mat (included) or with adapters on manikins.
Automated and User-activated Self-tests
- Daily Automatic Self-tests Tests: Tests internal circuitry, waveform delivery system, pads cartridge and battery capacity.
- Pads Integrity Test: Specifically tests readiness-for-use of pads (gel moisture).
- Battery Insertion Test: Upon battery insertion, extensive automatic self-tests and user-interactive test check device readiness.
- Status Indicator: Blinking green "Ready" light indicates ready for use. Audible "chirp" indicates need for maintenance.
Data Recording and Transmission
- Infrared: Wireless transmission of event data to a PC or Palm® PDA, using the IrDA protocol.
- Data Stored: First 15 minutes of ECG and the entire incident's events and analysis decisions.